



Next: The Laws of Quantum
Up: lect_notes
Previous: Probability
Contents
We have learnt that there is a wave associated with every particle.
This is not evident for macroscopic particles like a
bullet or an elephant because the wavelength
is extremely small. The wave nature becomes important
when dealing with microscopic particles like an electron for which the
wavelength can be of the order of 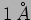 . We have
also learnt that this wave
. We have
also learnt that this wave 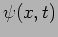 may be interpreted as the
probability amplitude and this wave is often referred to as the
wavefunction. The laws governing the evolution of the wavefunction
and
it interpretation are referred to as Quantum Mechanics.
may be interpreted as the
probability amplitude and this wave is often referred to as the
wavefunction. The laws governing the evolution of the wavefunction
and
it interpretation are referred to as Quantum Mechanics.
Subsections
Physics 1st Year
2009-01-06