



Next: Interpreting the electron wave
Up: Wave-particle duality
Previous: The Compton effect
Contents
In 1924 de Broglie first hypothesized that associated with every particle
there is a wave. In particular, we can associate a wave
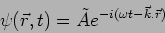 |
(17.11) |
With a particle which has energy
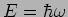 and momentum
and momentum
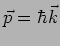 . The corresponding wavelength
. The corresponding wavelength 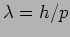 is
referred to as the de Broglie wavelength.
It should be noted that at any instant of time
is
referred to as the de Broglie wavelength.
It should be noted that at any instant of time 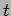 a particle has an unique well defined position
a particle has an unique well defined position 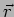 and momentum
and momentum
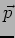 . Unlike the particle, at any time
. Unlike the particle, at any time 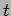 the wave
the wave
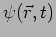 is defined all over space. This is the crucial difference
between a particle and a wave.
While the wave incorporates the particle's momentum, it does not
contain any information about the particle's position. This is an
issue which we shall return to when we discuss how to interpret the
wave associated with a particle.
is defined all over space. This is the crucial difference
between a particle and a wave.
While the wave incorporates the particle's momentum, it does not
contain any information about the particle's position. This is an
issue which we shall return to when we discuss how to interpret the
wave associated with a particle.
What is the dispersion relation of the de Broglie wave? A particle's
energy and momentum are related as
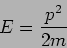 |
(17.12) |
which gives the dispersion relation
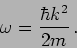 |
(17.13) |
Note that the relativistic relation
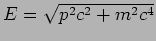 should be used at velocities comparable to
should be used at velocities comparable to 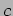 .
.
Problem: An electron is accelerated by a voltage
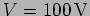 inside an electron gun. [a.] What is the de Broglie
wavelength of the electron when it emerges from the gun? [b.] When
do the relativistic effects become important? (Ans: a.
inside an electron gun. [a.] What is the de Broglie
wavelength of the electron when it emerges from the gun? [b.] When
do the relativistic effects become important? (Ans: a. 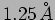 , [b]
, [b]
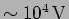 )
)
The wave nature of particles was verified by Davison and Germer in
1927 who demonstrated electron diffraction from a large metal
crystal. A pattern of maxima (Figure 17.3) is observed
when a beam of electrons is scattered from a crystal. This is very
similar to the diffraction pattern observed when X-ray are
scattered from a crystal. This clearly demonstrates that particles
like electrons also exhibit wave properties in some circumstances.
Problems
- Through how much voltage difference should an electron be
accelerated so that it has a wavelength of
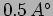 ?
?
- In a Compton effect experiment a photon which is scattered at
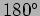 to the incident direction has half the energy of
the incident photon.
to the incident direction has half the energy of
the incident photon.
- a.
- What is the wavelength of the incident photon?
- b.
- What is the energy of the scattered photon?
- c.
- Determine the total relativistic energy of the scattered
electron.
- d.
- What is the momentum of the scattered electron?
- For what momentum is the de Broglie wavelength of an electron
equal to its Compton wavelength.




Next: Interpreting the electron wave
Up: Wave-particle duality
Previous: The Compton effect
Contents
Physics 1st Year
2009-01-06